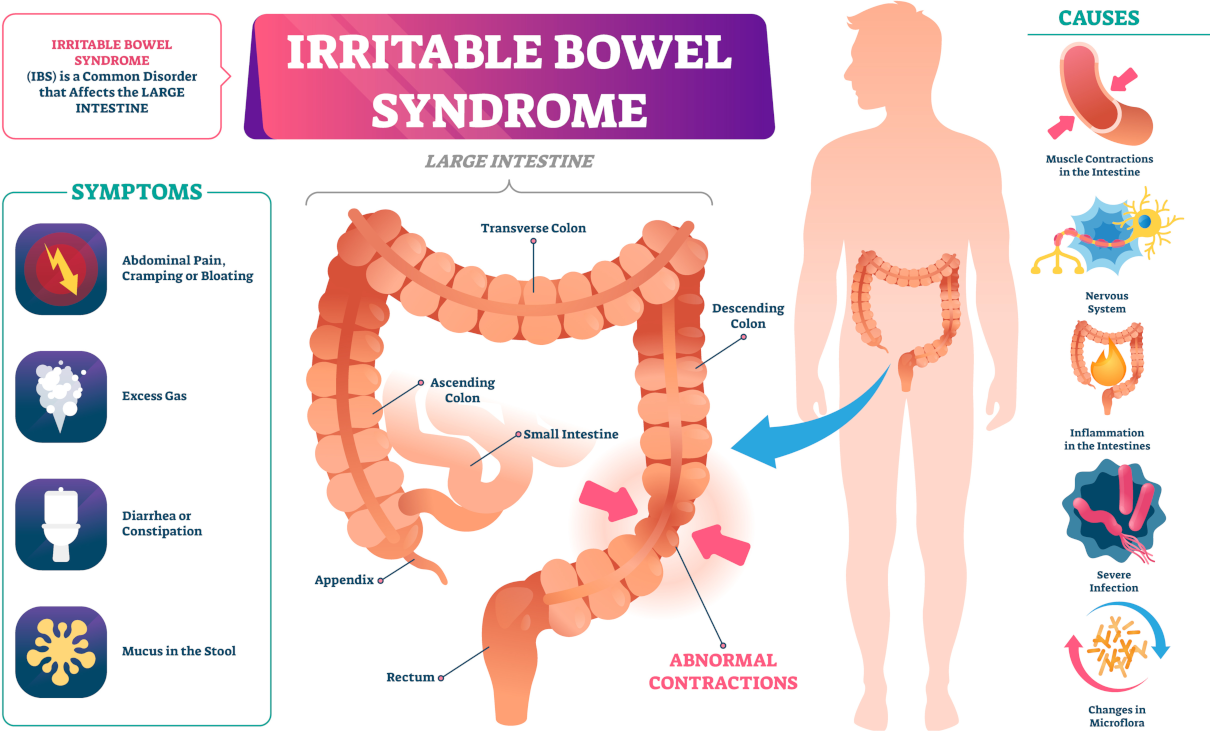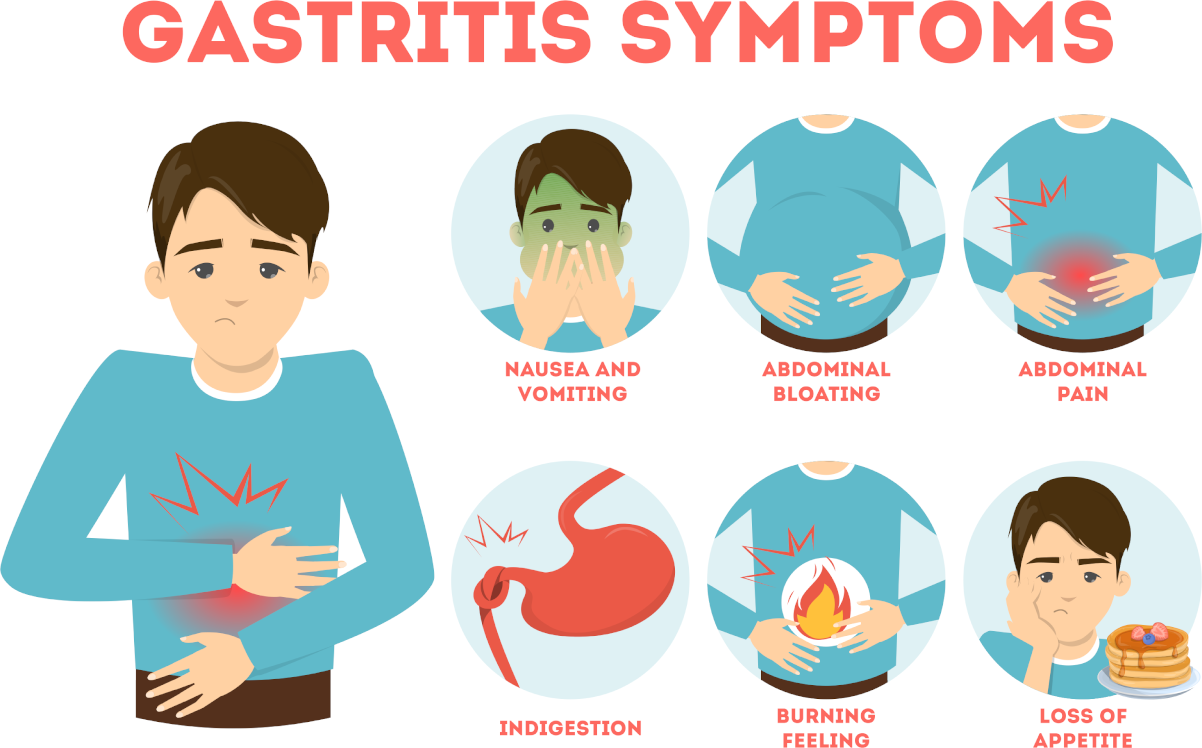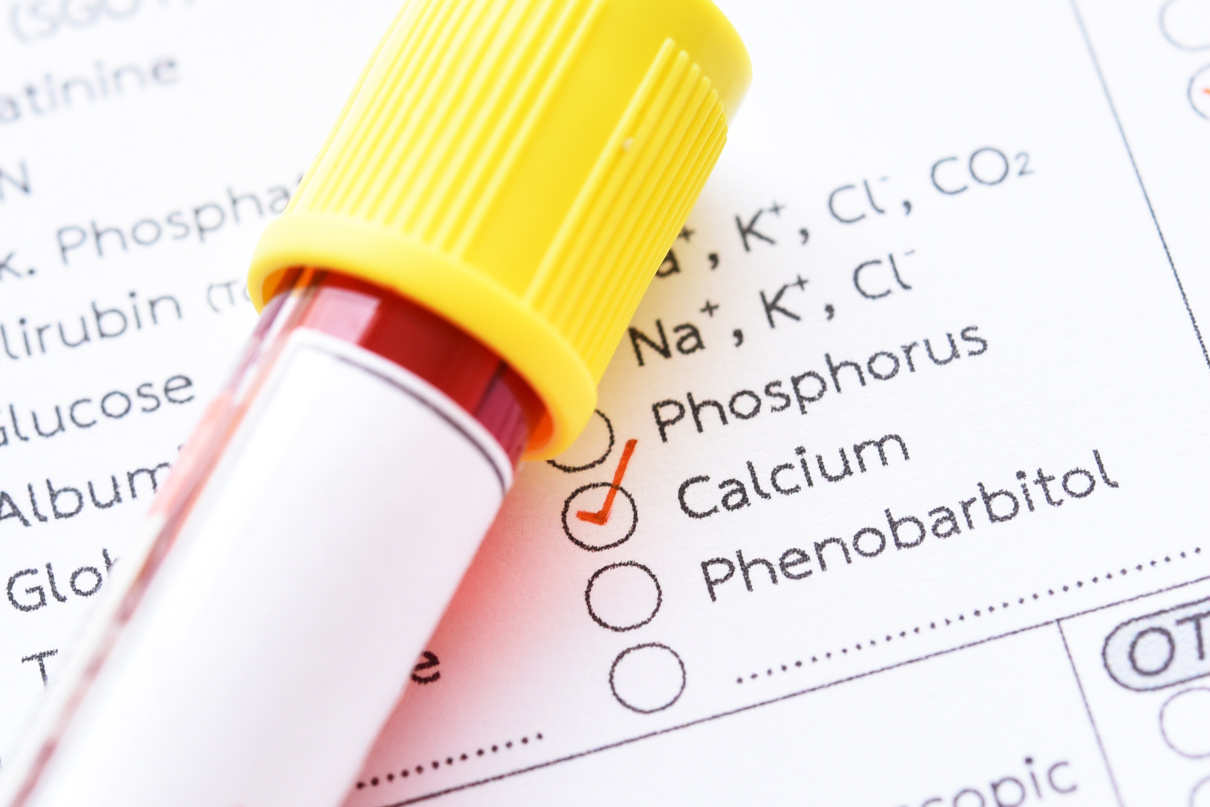
Serum calcium allows for the evaluation of the disorder of calcium metabolism. Besides, an increase in concentration can establish malignant tumours—osteolysis results from the release of peptides with actions similar to those of the thyrotropic hormone. Primary hyperfunction of parathyroid glands, overdosage of D vitamin, myeloma, and chronic enteritis (IVth stage) also impact adversely.
Medicinal hypocalcemia
A decrease in serum calcium concentration is possible at
- Hypo-activity of parathyroid glands (after surgical interventions, aplasia of parathyroid glands, autoimmune).
- Secondary hyper-function of parathyroid glands (insufficiency of D vitamin or resistance to it, chronic renal failure).
- And sequestration of calcium ions (acute alkalosis, phosphates rise, abundant transfusion of citrated blood).
- Osteomalacia of hypoalbuminemia, medicinal hypocalcemia (while receiving calcitonin, ethylene-diamine-tetraacetic acid, citrus, neomycin, fenobarbitalum).

Food intolerance test of 208 ingredients
This is our most comprehensive food and drink test. It analyses your client’s IgG antibody reactions to 208 food and drink ingredients. This test will highlight their food triggers and help you formulate an IgG-guided elimination diet together.
Characteristics of hypocalcemia
Below-normal serum calcium concentrations of 8.5-10.5 mg/dl (2.1-2.5 mmol / l characterise hypocalcemia. The introduction of routine biochemical studies into clinical practice has contributed to improving the diagnosis of disorders of calcium metabolism, predominantly asymptomatic hypercalcemia. Even though, on the whole, hypocalcemia is less common in ambulatory patients, it can occur more often in patients with malignant neoplasms and kidney diseases than hypercalcemia.
Our body maintains the serum calcium concentration within 2.2-2.6 mmol / l. However, ionised or protein-unbound calcium is slightly less than half of the total calcium. Ionised calcium, a physiologically active fraction, participates in many diverse metabolic processes. Significant changes in serum protein concentration, essentially albumin, disrupt whole serum calcium level. A simple way to correct the results of determining calcium in the blood is to increase the calcium concentration by 0.25 mmol / L while decreasing the serum albumin concentration by 10 g / L relative to the norm. Profound disturbances of calcium protein binding occur under the influence of changes in blood pH: an increase in pH leads to an increase in binding and, consequently, a decrease in the content of ionised calcium. This law explains the occurrence of symptoms of hypocalcemia in hyperventilating respiratory alkalosis.

Food allergy test
Do you experience reactions to foods and think an allergy might cause them? Do you experience hay fever-type symptoms, the cause of which is unknown to you? Allergies can be challenging to pinpoint. And it’s almost impossible to deal with an allergy without identifying what is causing the problem. This Food Allergy Test is a fast and reliable home-to-laboratory allergy testing service.